Abstract
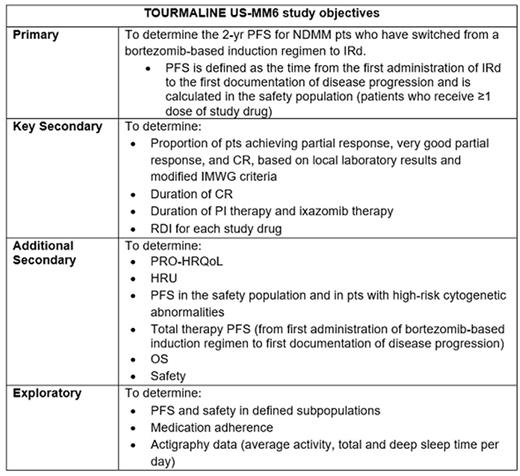
The use of proteasome inhibitor (PI)-based combinations and an increased focus on long-term treatment, including maintenance therapy, have improved long-term clinical outcomes for pts with NDMM. However, many pts discontinue therapy before disease progression due to toxicity, poor treatment adherence, or limited access to medical facilities. Hence, new, more tolerable and convenient treatment options are needed. Results from the global TOURMALINE-MM1 trial (NCT01564537) demonstrated the efficacy and tolerability of the oral PI ixazomib plus lenalidomide-dexamethasone (Rd) in pts with relapsed/refractory MM (Moreau et al. NEJM 2016;374:1621-34), and led to the approval of ixazomib, in combination with Rd, for the treatment of pts with MM who have received ≥1 prior therapy. Notably, TOURMALINE-MM1 showed no cumulative toxicity with prolonged IRd therapy. TOURMALINE US-MM6 (NCT03173092) is an open-label, single-arm, multicenter US study that is evaluating the effectiveness and safety of long-term use of the all-oral IRd regimen in the community practice setting in pts with NDMM who are switching from first-line induction treatment with a bortezomib-based triplet regimen. The study objectives are summarized in the Table.
The study will enroll ~160 NDMM pts over a 12-mo period at 25 US sites with a focus on community oncology practices, and is aiming to specifically include US ethnic populations who are currently under-represented in global clinical trials and for whom available data on the efficacy and safety of PI-based therapies are limited. Pts aged ≥18 yr who are transplant-ineligible (or for whom transplant is delayed for >24 mo), have received 3 cycles of bortezomib-based triplet induction therapy (bortezomib+cyclophosphamide+dexamethasone [VCd] or bortezomib+lenalidomide+dexamethasone [VRd]), and have no evidence of progressive disease (based on IMWG criteria) are eligible. Stem cell harvest and mobilization are allowed if clinically indicated. Pts receive ixazomib 4 mg on Days 1, 8, and 15, lenalidomide 25 mg on Days 1 to 21, and dexamethasone 40 mg (20 mg if aged ≥75 yr) on Days 1, 8, 15, and 22 of each 28-day cycle. IRd treatment is continued until progression or unacceptable toxicity leading to discontinuation or change in regimen, for a maximum of 26 cycles (over an estimated 24-mo period). After an end-of-study (EOS) assessment (within 30 days of last ixazomib dose), pts will enter a follow-up period for assessment of progression-free survival (PFS) and overall survival (OS) until disease progression/death, loss to follow-up, or termination of the study by the sponsor.
Baseline demographics, including race and ethnicity, and MM-specific characteristics and medical history are collected at enrollment. Response and disease progression are evaluated by the investigator on Day 1 of each cycle based on local laboratory results, using modified IMWG response criteria. Effectiveness of IRd therapy is assessed based on: 2-yr/total PFS, response rates, OS, relative dose intensity (RDI) received (for each study drug), duration of complete response (CR), duration of PI/ixazomib therapy, health-related quality of life (HRQoL), and health resource utilization (HRU) data. Safety of IRd is being assessed via reporting of serious and non-serious adverse events recorded from the first dose through 30 days after the last dose of study drug. Pt-reported outcomes (PRO)-HRQoL are being assessed at study enrollment, each treatment cycle, and the EOS assessment using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and the MM module (EORTC QLQ-MY20), and the Treatment Satisfaction Questionnaire for Medication (TSQM-9). Daily pt-reported medication adherence information is being collected via electronic devices, and actigraphy data are being captured by pts at home with a digital health wearable. HRU is being evaluated based on outpatient study site visits, hospital admissions, emergency department visits, and hospice care.
The TOURMALINE US-MM6 study aims to provide valuable data on the use of long-term therapy with the all-oral IRd regimen in the US community practice setting, including in pt groups who are currently under-represented in clinical trials. Study sites are currently being selected; pt recruitment commenced in September 2017. Further details regarding study rationale and protocol will be provided.
Rifkin: Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; McKesson Specialty Health: Employment; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Boccia: Celgene: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; DSI: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Center for Cancer and Blood Disorders: Research Funding; Genentech: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Kambhampati: Takeda: Consultancy, Honoraria. Richter: Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau. Usmani: Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Array BioPharma: Honoraria, Research Funding; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Honoraria, Research Funding; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Speakers Bureau; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Aiello: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy, Honoraria. Sickler: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Schoeneich: Neurocrine Biosciences: Equity Ownership; Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Wang: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Zelt: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Whidden: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Dollar: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Niculescu: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Noga: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment; Takeda Pharmaceutical Company Limited: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.